
In the case that this is not true, a current can be passed between two electrodes in order to induce the reaction by creating an excess of negative charge at the cathode and an absence of negative charge(effectively a positive charge) on the anode.īy extension, passing an adequate current through two electrodes in water will result in the oxidation of oxygen and the reduction of hydrogen and therefore the production of both gases.ĮDIT: after reading your reply to /u/defaultprimate I see that you are asking why O 2 does not also oxidise from H 2O. Basically, any redox reaction will occur spontaneously if the reduction potential of the cathode is greater than the reduction potential of the anode. This can be induced by electrolysis, yes. Pastebin / / IDEOne Revert to older template If you have trouble with spacing, add some blank spaces with įor more commands, please see User Moderation

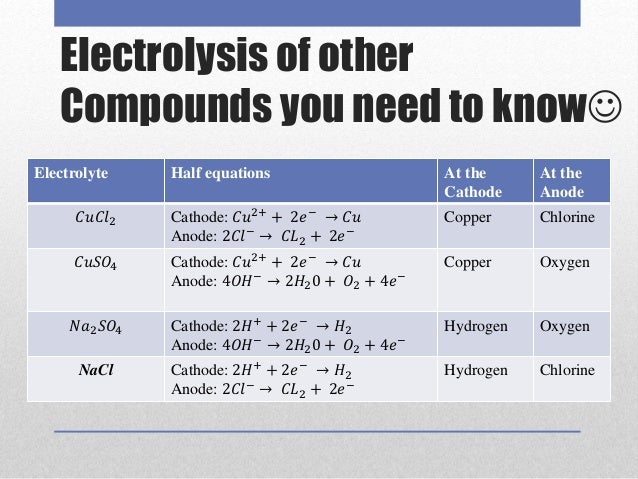
Cathode reaction in electrolytsis of cucl2 how to#
Please contact u/Oryv if the bot malfunctions, or if you have questions on how to use it.
Cathode reaction in electrolytsis of cucl2 code#
Simply mention the bot in a comment and put the expressions that need to be rendered in code blocks, and u/LaTeX4Reddit will respond. To use LaTeX on this subreddit, use u/LaTeX4Reddit. Accept answers at your own risk.įor citation questions, check the Purdue Online Writing Lab Keep in mind that we do not and will not have any sort of vetting procedure for responders. This includes asking for "likes," page views, or similar things. Offers or solicitations of payment in any form. Requests for help with cheating, plagiarism, or other violations of academic integrity violations of copyright or terms of use or other illegal or unethical activities. Posts tagged "urgent," "ASAP," "important," "due in an hour," etc. "Do this for me" posts such as posting of quizzes or lists of questions.

When your question has been answered, please edit the post's flair to " ✓ Answered".Ĭopied questions without context or explanation. What does your instructor want you to accomplish? Still acceptable…, but preferably not: Grade 1-6 (Pri) Grade 7-10 (Sec) Grade 11-12 (Pre-Uni) University/College ❗️ READ THE RULES BEFORE POSTING


 0 kommentar(er)
0 kommentar(er)
